Abstract
Background
CD123 (IL-3 receptor alpha-chain) is a therapeutic target for hematological malignancies based on high expression levels in acute myeloid leukemia (AML), blastic plasmacytoid dendritic cell neoplasm (BPDCN), and other cancers. The anti-CD123 antibody-drug conjugate (ADC), IMGN632, comprises a humanized monoclonal antibody covalently linked to a DNA - alkylating cytotoxic payload which is currently in phase 1 evaluation for relapsed/refractory CD123-positive hematological malignancies (NCT03386513). Novel approaches to enhance the efficacy of ADCs are of significant therapeutic interest. Our laboratory has previously demonstrated that the Poly ADP Ribose (PARP) inhibitor, olaparib, synergistically enhances the activity of the CD33-targeted ADC, IMGN779, in preclinical AML models (Portwood S et al, ASH 2016). Based on the hypothesis that PARP inhibition will synergize with DNA damaging mechanism of IMGN632, we investigated the ability of olaparib and other PARP inhibitors (PARPi) in clinical development (talazoparib, niraparib, rucaparib, and veliparib) to enhance the therapeutic efficacy of IMGN632 across diverse human AML cell lines and primary relapsed/refractory AML samples.
Materials and Methods
CD123 expression on human AML cell lines (HEL, HL60, MV411, Molm13, EOL-1, THP-1, and Kasumi-1) was quantified by flow cytometry using QuantriBrite beads. AML cells were continuously cultured for 72-96 hours with varying doses of IMGN632 (range 100pM - 100nM) and specific PARP inhibitors (range 100pM -15μM) alone and in combination. Cell viability was measured using a WST-8 colorimetric assay. Primary clinically annotated CD123+ AML cells from patients with relapsed/refractory disease were obtained under IRB-approved protocols from the Roswell Park Hematologic Procurement Shared Resource and cultured short-term in the presence of multiple cytokines plus IMGN632 +/- PARP inhibitors. Apoptosis (Annexin V/PI), cell cycle, and DNA damage (H2AX) were evaluated by flow cytometry. Additive vs. synergistic effects were determined by combination indices using Compusyn software. PARP trapping was evaluated by Western blot analysis in nuclear lysates obtained from IMGN632 +/- PARP inhibitors treated AML cells.
Results
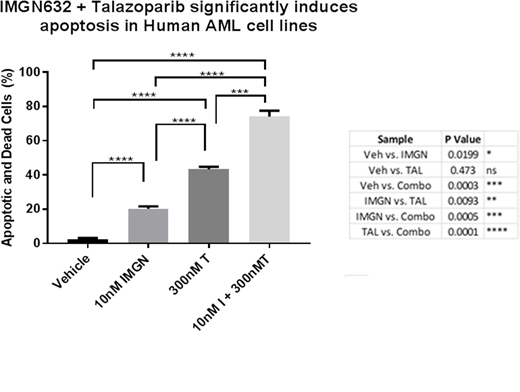
High expression levels of CD123 (range 937 - 2231 CD123 molecules/cell) were detected on multiple human AML cell lines (HEL-luc, MV411, Molm13, EOL-1, and THP-1) relative to unstained negative controls. Western blot analysis of nuclear lysates from AML cells demonstrated that all PARP inhibitors had varying degrees of PARP trapping on DNA. Continuous single agent 5-day treatment with all tested PARP inhibitors resulted in dose dependent in vitro inhibition of AML cell line growth with IC50 values ranging from 360 nM (talazoparib, most potent) to 78uM (veliparib, least potent). Combination therapy using PARP inhibitors (doses ranging from 300nM - 15uM) and IMGN632 (10nM) consistently resulted in enhanced anti-leukemic effects over monotherapy (Figure 1 for example). Synergistic anti-proliferative effects were obtained across all tested AML cell lines (n=5) with combination indexes ranging from 0.3-0.7 by Compusyn analysis. Combination therapy correlated with enhanced DNA damage, tumor cell apoptosis, and cell cycle arrest of AML cells. Moreover, IMGN632 and PARPi (olaparib or talazoparib) resulted in single agent activity against clinically annotated primary relapsed/refractory AML patient samples with evidence of synergistic effects when combined in vitro.
Conclusions
Addition of PARP inhibitors to IMGN632, a novel anti-CD123 antibody-drug conjugate, further enhances DNA damage effects and consistently results in synergistic in vitro anti-leukemic effects across multiple CD123+ AML cell lines and primary AML patient samples. Further studies investigating this novel combinatorial approach in specific molecular subtypes of AML with variable baseline sensitivities to PARPi are currently ongoing. Our results strongly support future investigation of PARPi as a novel class of agents with the potential to significantly enhance the efficacy of DNA-alkylating ADCs and/or cytotoxic chemotherapy for hematological malignancies.
Sloss:ImmunoGen: Employment. Watkins:ImmunoGen Inc.: Employment. Kovtun:ImmunoGen Inc.: Employment. Adams:ImmunoGen Inc.: Employment. Wang:Abbvie: Consultancy, Membership on an entity's Board of Directors or advisory committees; Novartis: Speakers Bureau; Abbvie: Consultancy, Membership on an entity's Board of Directors or advisory committees; Amgen: Consultancy; Novartis: Speakers Bureau; Jazz: Speakers Bureau; Jazz: Speakers Bureau; Pfizer: Consultancy, Membership on an entity's Board of Directors or advisory committees; Amgen: Consultancy; Pfizer: Consultancy, Membership on an entity's Board of Directors or advisory committees.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal